Clinical studies with green tea
The Asian paradox
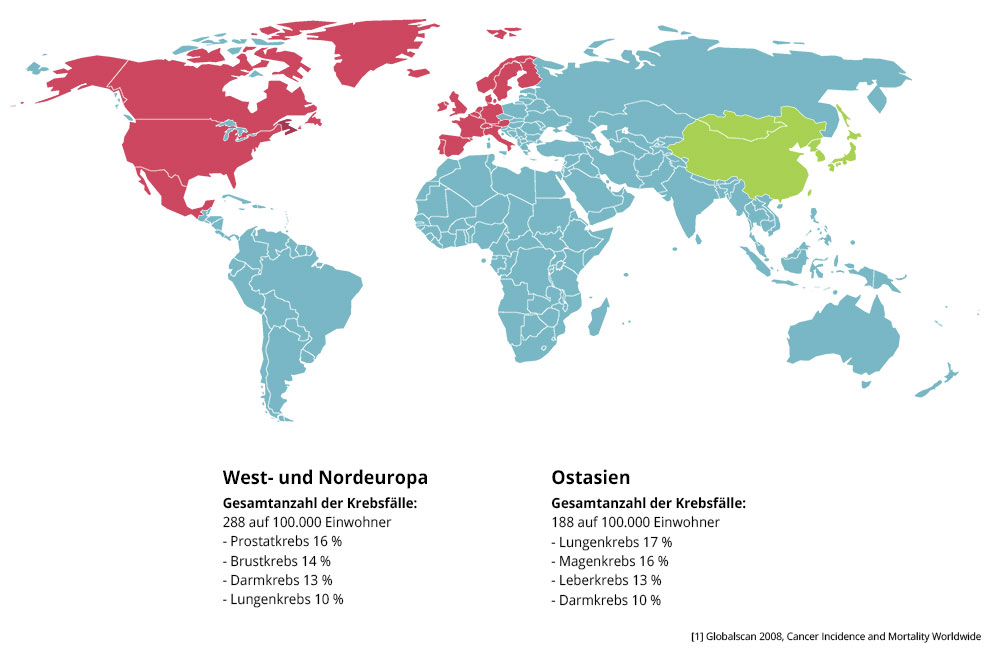
In Asia green tea is an inherent part of the culture. In Japan, according to prefectures, people drink up to 1,5 liter/day. The already implemented, over 11 years running study in japan called Ohsaki-Study with 40.530 adults in the age between 40 to 79 years showed, that green tea has positive cardiovascular effects what leads into a life-extending outcome. The dying-rate for male participants, which drunk more than five cups on a daily base, sunk to 12 percent, for women down to 23 percent.
The renowned University of Yale evaluated over 100 studies with green tea in 2006. The researchers wanted to find out, why in Asia with the highest consume of cigarettes worldwide the cardiovascular diseases and cancer is lower than in western countries. Prof. Dr. Bauer Sumpio, head of the study, evaluated more than 100 experimental and clinical studies about green tea.
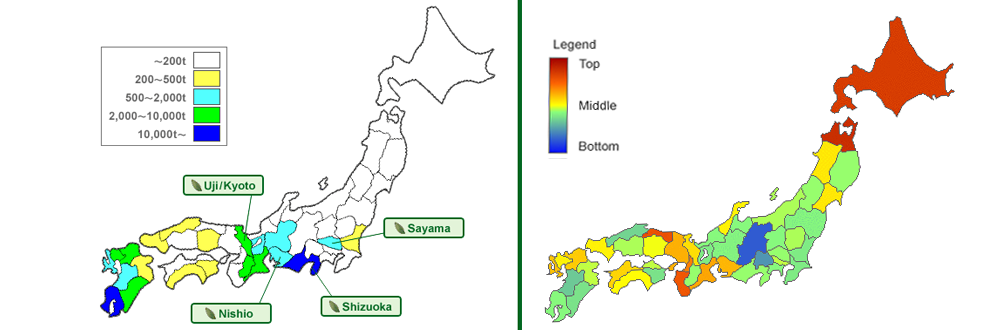
Interestingly there are no comparable cancer-rates in other countries with a high consumption of tea. It’s noticeable that especially regions in japan, where a big amount of green tea is produced (left), have a lower lung cancer disease-rate (right) than others. Most of all regions, which produce and consume plenty of green tea, show the lowest disease-rates. It´s really difficult to determine causality on the basis of many factors (City- vs. land population, different eating habits, etc.). However it is striking that the consumption of green tea plays a crucial role.
Furthermore there are studies, which prove the assimilation of cardiovascular diseases of immigrated Japanese in the USA. The change of consumption-habits seems to have a big impact on the health.
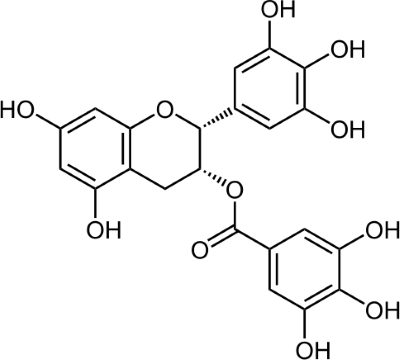
EGCG – the active compound in green tea
In the recent years scientists noticed, that EGCG has a significant positive influence on many incurable diseases like Alzheimer´s, Parkinson´s, Huntington´s chorea or ALS – without the harmful secondary effects of traditional medications or treatments. In several clinical experiments EGCG was able to prevent brain cells from mortifying and thus operated “neuroprotective”.
A placebo-controlled clinical pilot study, carried out by the University Clinic Boston (USA), with 42 patients with coronary heart diseases showed, that the endothelium function was improving after an administration of 300mg EGCG. Vascular dilation was measured with ultrasound. The improved endothelium function of the vessels led to an improvement of the blood pressure readings.
Another EGCG-study with 87 patients from Wales in the year 2008 was able to prove a significant effect on the diastolic blood pressure.
A study of the University of Dundee from November 2014 about the application of EGCG in combination with Cysteamin (Thioethanol-amin) revealed that 90 % of the patients showed an improvement of inflammation-symptoms. The study showed a noticeable increased effectivity with Cysteamin in combination with EGCG. EGCG in combination with Cysteamin is going to improve the therapy of mucoviscidosis in the opinion of the scientists.
The case of Prof. Hunstein
Some individual cases caused sensations in the recent years. The highly esteemed hematologist from Heidelberg, Prof. Dr. Werner Hunstein developed 2003 a case of amyloidosis, a rare and always deadly disease, which causes proteins to thicken and deposit. Crucial cases of the disease cause the cardiac septum and the tongue to thicken, so that the patient becomes weak and vulnerable. Currently amyloidosis is treated with chemotherapy. This radical treatment extends the lifespan generally only about a few months.
This was the case for the seriously ill patient Prof. Dr. Hunstein. The chemotherapy in the year 2006, which took all his physical strength, could extend his expectancy only a few months. As he was told about the first results of a research group from the Max-Delbrück Center for molecular medicine regarding EGCG he decided to do a comprehensive self-experiment. He drank up to 2 liter of very intense green tea each day.
Just after he started his „Green-Tea-Therapy“ he became witness of the unexpected. In a short time his symptoms of disease were completely reduced. This case created an international stir. Many newspapers reported about his case.
How was that possible?
The EGCG seemingly break all devastating proteins down, which were deposit in the whole body. It was even determined, that the thickness of the cardiac septum was measureable reduced. The consumption of green tea afforded Prof. Dr. Hunstein another 5 ½ years without afflictions. This case is all the more astounding, that till this day there is no therapy for amyloidosis. Amyloidosis is still an immedicable disease, which is based on the misfolding of proteins. After a diagnosis the expectation of life is commonly less than 6 months.
A detailed report of Prof. Hunstein can be found here: Website von Prof. Hunstein
Inhibition of protein-misfolding
EGCG within the green tea has a natural effect on the arrest of protein-misfolding. It operates on a cellular level and prevents the fatal concatenation process of the proteins. So the irreversible dying of brain cells can be prevented. EGCG can destroy already linked proteins and stop the process of concatenation. Toxic, misfolded proteins are turned into harmless aggregate, so-called “oligomere”. An important aspect is the excellent tolerance of green tea. The beverage, that was tested over thousands of years on humans, shows even with high doses (>10 cups per day) no significant side effects.
Scientists achieved new insights the recent years, that prove, that amyloidosis, Down´s syndrome, Alzheimer´s, Parkinson´s and many other neurodegenerative diseases are based on the same fundamental pathological process: Proteins are chaining up in an accelerating way and destroying cells in the brain and organs. Scientists talk about a “deadly cascade”, which evolves unnoticed over the years.
The dying of brain cells (Neurons) goes along with forgetfulness and the loss of personality with the Alzeimer´s disease, the tremor with Parkinson´s as well as learning disability with Down´s syndrome. The search for a neuroprotective substance was a long one. Through the detection of EGCG scientists were able to find a substance with the needed effects.
Great success of EGCG for the therapy of neurological deficits
A study of the university clinic Barcelona was possible to determine a cognitive improvement on young adults with Down´s syndrome, as they got treated with EGCG. Down´s syndrome is a disease based on genetic mechanisms, which comes hand in hand with a learning disability. Young people with Down´s syndrome learn very slowly. The massive and early appearance of the Alzheimer´s disease in this connection is a major problem.
As part of this study young patients were delivered EGCG in a form of a health supplement. It was immediately after the beginning of the therapy a positive effect noticeable, which was proven with many measurement series. A key factor is that the families of the patients could notice an explicit improvement in daily routines and the enhancement of livability. After the therapy stop within the study, an aggravation of the cognitive performance took place, which leads to the conclusion, that the intake of EGCG is needed on a daily base.
Green tea resp. EGCG is the first actual therapeutic approach, which has a measurable positive effect on neurological deficits. Crucial is the fact, that the neurological deficits on Down´s syndrome are caused by misfolded proteins. EGCG has a wide range of protein-based activity. EGCG can therefore be used on many other neurological or in general protein-misfolding-based diseases.
An overview about the diverse field of application of EGCG is listed here:
List of clinical studies according to the register of U.S.-Department of Health & Human Services (Dec. 14)
| Object of investigation | Topic | Year | Contact | Country | Code | ||
|---|---|---|---|---|---|---|---|
| 1 | Relapsing-remitting Multiple Sclerosis | Neuro | 2007 | 2013 | Anja Mähler | Deutschland | NCT00525668 |
| 2 | Chronical Progressive Multiple Sclerosis | Neuro | 2008 | – | Prof. Dr. Paul Friedemann | Deutschland | NCT00799890 |
| 3 | Alzheimer’s Disease | Neuro | 2009 | – | Friedemann Paul, MD | Germany | NCT00951834 |
| 4 | Duchenne Muscular Dystrophy | Neuro | 2010 | – | Friedemann Paul, MD | Germany | NCT01183767 |
| 5 | Multiple Sclerosis | Neuro | 2014 | – | Dr. Orhan Aktas | Germany | Geplant |
| 6 | Multiple Sclerosis Pilot Study: Safety Study | Neuro | 2009 | 2013 | Jesus F Lovera, MD | U.S. | NCT00836719 |
| 7 | Multiple Sclerosis: Safety and Brain Protection | Neuro | 2013 | – | Jesus Lovera, MD | U.S. | NCT02011451 |
| 8 | Multiple Sclerosis | Neuro | 2013 | – | Jesus F Lovera, MD | U.S. | NCT02011451 |
| 9 | Cognitive Function; Mood | Neuro | 2009 | 2012 | Crystal Haskell | U.K. | NCT00981292 |
| 10 | Cognition, stress, brain function and cardiovascular function | Neuro | 2009 | – | Con Stough | Australia | ACTRN12609000646246 |
| 11 | Cognitive Performance in Fragile-X (including Down Syndrome) | Neuro | 2013 | – | De la Torre Rafael, PharmD | Spain | NCT01855971 |
| 12 | Huntington Disease | Neuro | 2011 | 2014 | Josef Priller, MD | Germany | NCT01357681 |
| 13 | Down Syndrome | Neuro | 2011 | 2013 | Rafael De la Torre Fornell, PhD | Spain | NCT01394796 |
| 14 | Multiple System Atrophy | Neuro | 2014 | – | Johannes Levin, MD | Germany | DRKS00005610 |
| 15 | Huntington´s Disease | Neuro | 2011 | – | Friedemann Paul, MD | Germany | 2010-023941-31 |
| 16 | Duchenne Muscular Dystrophy | Neuro | 2010 | – | Friedemann Paul, MD | Germany | 2009-016482-28 |
| 17 | Multiple Sclerosis | Neuro | 2011 | 2014 | Jesus Lovera MD | U.S. | NCT01451723 |
| 18 | Multiple System Atrophy | Neuro | 2013 | – | Johannes Levin, MD | Germany | NCT02008721 |
| 19 | Down syndrome | Neuro | 2012 | – | Rafael De la Torre Fornell, PhD | Spain | NCT01699711 |
| 20 | Multiple Sclerosis, Relapsing-Remitting | Neuro | 2011 | 2013 | Friedemann Paul, MD | Germany | NCT01417312 |
| 21 | Parkinson’s Disease | Neuro | 2007 | 2011 | Piu Chan, MD, PhD | China | NCT00461942 |
| 22 | Alzheimer´s disease (early stage) | Neuro | 2009 | – | Friedemann Paul, MD | Germany | 2009-009656-20 |
| 23 | Primary and secondary progressive forms of multiple sclerosis | Neuro | 2008 | – | Friedemann Paul, MD | Germany | 2008-005213-22 |
| 24 | Relapsing-remitting multiple sclerosis ICD classification: G35.1 | Neuro | 2007 | 2014 | Friedemann Paul, MD | Germany | 2006-006323-39 |
| 25 | Cardiac amyloid Light-chain amyloidosis TAME-AL | Amyloidose | 2013 | – | Stefan Schönland, MD | Germany | NCT02015312 |
| 26 | Primary Amyloidosis of Light Chain Type | Amyloidose | 2012 | – | Giovanni Palladini, Dr. | Italy | NCT01511263 |
| 27 | Cardiac transthyretin amyloidosis | Amyloidose | – | 2012 | PD Dr med A. V. Kristen | Germany | – |
| 28 | Cardiac amyloid light-chain amyloidosis | Amyloidose | 2012 | – | Stefan Schönland | Germany | ISRCTN68399350 |
| 29 | Advanced Non-Small Cell Lung Cancer | Krebs | 2008 | 2013 | Dr. Glenn Mills | U.S. | NCT00707252 |
| 30 | Small Cell Lung Carcinoma | Krebs | 2011 | – | Xindong Sun, M.D. | China | NCT01317953 |
| 31 | Lung Cancer Prevention | Krebs | 2006 | 2013 | Iman Hakim, MD, PhD, MPH | U.S. | NCT00363805 |
| 32 | Lung Cancer; Precancerous Condition; Tobacco Use Disorder | Krebs | 2008 | 2012 | Stephen Lam, MD | U.S. | NCT00611650 |
| 33 | Lung Cancer; Tobacco Use Disorder | Krebs | 2007 | 2012 | Stephen Lam, MD | U.S. | NCT00573885 |
| 34 | Breast Cancer | Krebs | 2008 | 2012 | Gary Burton, M.D. | U.S. | NCT00676793 |
| 35 | Estrogen Receptor-negative Breast Cancer; Progesterone Receptor-negative Breast Cancer; Stage I Breast Cancer; Stage II Breast Cancer; Stage IIIA Breast Cancer; Stage IIIB Breast Cancer | Krebs | 2007 | 2014 | Dawn Hershman | U.S. | NCT00516243 |
| 36 | Cervical Cancer; Cervical Intraepithelial Neoplasia Grade 1 | Krebs | 2006 | 2014 | Francisco Garcia | U.S. | NCT00303823 |
| 37 | Vulval intraepithelial neoplasia (VIN) | Krebs | 2014 | – | University of Birmingham | U.K. | 2013-003107-19 |
| 38 | Prevention of Breast cancer | Krebs | 2009 | – | Dr Min Zhang | Australia | ACTRN12609000098235 |
| 39 | Stage I Prostate Cancer; Stage IIA Prostate Cancer; Stage IIB Prostate Cancer | Krebs | 2011 | 2012 | Sanjay Gupta | U.S. | NCT01340599 |
| 40 | Stage I Prostate Cancer; Stage IIA Prostate Cancer; Stage IIB Prostate Cancer | Krebs | 2013 | – | Robert Abouassaly, MD | U.S. | NCT01928485 |
| 41 | Prostate Cancer | Krebs | 2008 | 2012 | Jerry W McLarty, Ph.D. | U.S. | NCT00676780 |
| 42 | Prostatic intraepithelial neoplasia | Krebs | 2007 | – | SOFAR SPA | Italy | 2007-000759-32 |
| 43 | Adenocarcinoma of the Prostate; Stage I Prostate Cancer; Stage II Prostate Cancer | Krebs | 2007 | 2013 | Frederick Ahmann | U.S. | NCT00459407 |
| 44 | Prostatic Hyperplasia | Krebs | 2008 | – | Nagi Kumar, PhD | U.S. | NCT00596011 |
| 45 | Precancerous Condition; Prostate Cancer | Krebs | 2005 | – | Jackilen Shannon, PhD | U.S. | NCT00253643 |
| 46 | Localized cancer of the prostate | Krebs | 2007 | – | University of Oslo, institute for basic medical sciences, department of nutrition | Norway | 2006-006679-18 |
| 47 | Patients with first prostate biopsy with diagnosis of ASAP (Atypical Small Acinar Proliferation) or multifocal (≥2 positive samples) HGPIN (High-Grade Prostatic Intraepithelial Neoplasia) | Krebs | 2009 | – | AZIENDA SANITARIA OSPEDALIERA | Italy | EUCTR2009-014548-13-IT |
| 48 | Bladder Cancer | Krebs | 2004 | 2012 | Arie Belldegrun, MD | U.S. | NCT00088946 |
| 49 | Stage I Bladder Cancer; Stage II Bladder Cancer; Stage III Bladder Cancer | Krebs | 2008 | 2013 | Tracy Downs | U.S | NCT00666562 |
| 50 | Cytotoxic Effects of Chemotherapeutic Agents in Human Urothelial Carcinoma Cells | Krebs | 2013 | – | Kuo-How Huang, M.D.,Ph.D. | Taiwan | NCT01993966 |
| 51 | Unspecified Adult Solid Tumor, Protocol Specific | Krebs | 2004 | 2010 | H. H. Sherry Chow, PhD | U.S. | NCT00091325 |
| 52 | Leukemia | Krebs | 2005 | 2013 | Jose F Leis, MD | U.S. | NCT00262743 |
| 53 | Non-melanomatous Skin Cancer | Krebs | 2000 | 2014 | Frank L. Meyskens, MD | U.S. | NCT00005097 |
| 54 | Unspecified Adult Solid Tumor, Protocol Specific | Krebs | 2000 | 2014 | H. H. Sherry Chow, PhD | U.S. | NCT00091325 |
| 55 | Multiple Myeloma and Plasma Cell Neoplasm; Precancerous Condition | Krebs | 2009 | – | Jeffrey A. Zonder, MD | U.S. | NCT00942422 |
| 56 | Plasma Cell Neoplasm Or Smoldering Multiple Myeloma | Krebs | 2011 | – | Jeffrey Zonder MD | U.S. | NCT01589887 |
| 57 | Ductal Carcinoma in Situ | Krebs | 2010 | – | Nora Jaskowiak, MD | U.S. | NCT01060345 |
| 58 | Leiomyoma | Krebs | 2011 | 2013 | Ayman Al-Hendy, MD, PhD | U.S. | NCT01311869 |
| 59 | Mild to Moderately Active Ulcerative Colitis | Verdauung | 2008 | 2012 | Gerald W Dryden, MD | U.S. | NCT00718094 |
| 60 | Advanced Colorectal Adenomas; Adenocarcinoma of the Colon; Stage I Colon Cancer; Stage II Colon Cancer; Stage III Colon Cancer | Verdauung | 2012 | – | Richard V. Benya | U.S. | NCT01606124 |
| 61 | Barrett Esophagus | Verdauung | 2005 | 2014 | Charles Lightdale | U.S. | NCT00233935 |
| 62 | Bowel health | Verdauung | 2012 | 2013 | Ms Jane Upton | Australia | ACTRN12613000097741 |
| 63 | Diabetes Mellitus; Diabetic Nephropathy; Arterial Hypertension | Adipositas | 2010 | 2012 | Jose b Lopes de Faria, M.D. | Brazil | NCT01130727 |
| 64 | Obesity; Type 2 Diabetes Mellitus | Adipositas | 2009 | 2009 | Ellen E Blaak, PhD, Prof | Netherlands | NCT00867555 |
| 65 | Lipid metabolism, creation of health foods (ice cream) | Adipositas | 2014 | – | Hisashi Imbe | Japan | JPRN-UMIN000015009 |
| 66 | Obesity | Adipositas | 2005 | 2014 | Conrad Earnest, PhD | U.S. | NCT00153790 |
| 67 | Obese women and obese related hormone peptides | Adipositas | 2014 | – | Chung-Hua Hsu, PHD | Taiwan | NCT02147041 |
| 68 | Albuminuria, Diabetic Nephropathy | Adipositas | 2013 | – | Cynthia Borges, MD | Brazil | NCT01923597 |
| 69 | Lipid metabolism, and creation of health foods processed from tea | Adipositas | 2013 | – | Hisashi Imbe | Japan | JPRN-UMIN000011901 |
| 70 | Obesity | Adipositas | 2012 | – | Margriet S Westerterp-Plantenga, Prof. Dr. | Netherlands | NCT01556321 |
| 71 | Diabetes, Postprandial lipemia | Adipositas | 2012 | – | Stephen H Boutcher | Australia | ACTRN12612000188831 |
| 72 | Vascular problems related to a high fat meal | Adipositas | 2012 | – | Yati Bouthcer | Australia | ACTRN12612000179831 |
| 73 | Obesity | Adipositas | 2007 | 2009 | Arne Astrup, Professor | Denmark | NCT00611416 |
| 74 | Obesity, Insulin resistance | Adipositas | 2010 | – | Steve Boutcher | Australia | ACTRN12610000965000 |
| 75 | High visceral fat | Adipositas | 2009 | 2009 | Ying Zhang | China | ChiCTR-TRC-10000872 |
| 76 | Hypertension; Insulin Resistance; Obesity; Type 2 Diabetes | Adipositas | 2007 | 2014 | Michael J Quon, MD, PhD | U.S. | NCT00434499 |
| 77 | Cardiovascular disease, Overweight and obesity | Adipositas | 2009 | – | Yati Boutcher | Australia | ACTRN12609000509268 |
| 78 | HIV-1 | Virus | 2011 | – | Christina L Nance, PhD | U.S. | NCT01433289 |
| 79 | HIV Infection: Safety and Toxicity | Virus | 2011 | – | Christy Nance PhD | U.S. | NCT01433289 |
| 80 | Virus Reactivation in Remission Patients (NPC) | Virus | 2012 | 2013 | Jin Ch Lin, MD PHD | Taiwan | NCT01744587 |
| 81 | Chronic Hepatitis C, oxidative stress | Virus | 2009 | 2013 | Roy L Hawke, PhD, PharmD | U.S. | NCT01018615 |
| 82 | Epidermolysis Bullosa Dystrophica | Haut | 2009 | 2014 | Christine Chiaverini, PhD | France | NCT00951964 |
| 83 | Acne Vulgaris | Haut | 2010 | 2012 | Dae Hun Suh, M.D., Ph.D. | Korea | NCT01687556 |
| 84 | Lamellar Ichthyosis | Haut | 2010 | 2010 | Chiaverini Christine, Dr | France | NCT01222000 |
| 85 | Genital Warts; Perianal Warts | Haut | 2010 | 2010 | Medigene AG | Germany | NCT01082302 |
| 86 | Condylomata Acuminata | Haut | 2007 | – | Medigene AG | Germany | NCT00449982 |
| 87 | Radio Dermatitis and Radiation Mucositis | Haut | 2011 | – | Jinming Yu, M.D. | China | NCT01481818 |
| 88 | Dystrophinopathies (Duchenne/Becker muscular dystrophy) | Sonstige | 2011 | 2014 | Yoshiko Tsuchie | Japan | JPRN-UMIN000005945 |
| 89 | Cardiovascular Diseases | Sonstige | 2012 | 2013 | Mario Lorenz, PhD | Germany | NCT01662232 |
| 90 | Atherosclerosis | Sonstige | 2008 | 2011 | Amir Lerman, MD | U.S | NCT00865787 |
| 91 | Cystic Fibrosis | Sonstige | 2009 | – | Eitan Kerem, MD | Israel | NCT00889434 |
| 92 | Glaucoma and ocular hypertension | Sonstige | 2006 | 2007 | Policlinico Gemelli. Clinical Trial Center, universita cattolica | Italy | EUCTR2006-005943-27-IT |
| 93 | Osteoarthritis | Sonstige | 2012 | 2013 | Dr.gholamreza Hatam | Iran | IRCT201307188300N2 |
| 94 | Chronic peridontitis | Sonstige | 2014 | – | ATHIRA P R | Indien | CTRI/2014/08/004925 |
| 95 | Endometriosis | Sonstige | 2011 | – | P.G.A. Hompes | Netherlands | NTR2760 |


